|
|
Progress in the study of tin sites and their reversible interconversion in zeolites |
| Author: |
|
Update time: |
2018-06-15 |
|
 |
Professor Feng Deng and Professor Jun Xu at Wuhan Institute of Physics and Mathematics (WIPM), Chinese Academy of Sciences have made new progress with collaboration with Professor Fengshou Xiao’s group from Zhejiang University in the direct observation of tin sites and their reversible interconversion in zeolites. The work was published in the journal Communication Chemistry.
Biomass is the only renewable and inexhaustible carbon source on earth. The great challenge in modern chemistry is to develop new catalysts and processes for selective conversion of biomass to produce fuels and fine chemicals. Tin-substituted β zeolite (Sn-β) being as an important solid Lewis acid catalyst exhibits unparalleled catalytic performance in transformation of biomass and biomass-derived feedstocks. Due to the low concentration of Sn loading (usually lower than 2 wt%) and similar coordination environment of different Sn sites, unambiguous spectroscopic discrimination of Sn sites is difficult. Rational design of Sn-containing zeolites with higher activity and selectivity can only be achieved by fully understanding the structure and nature of their active sites.
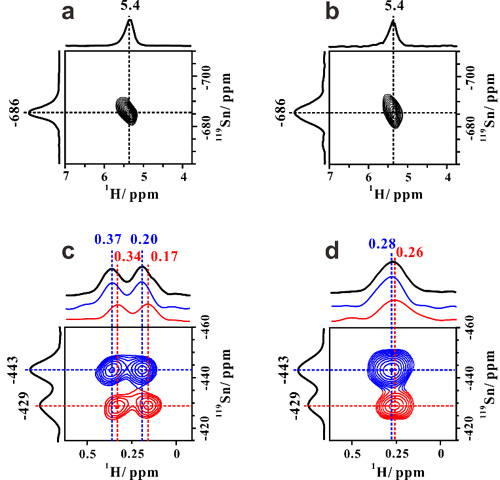
In previous work (Angew. Chem. Int. Edit. 2016, 55, 15826,ACS Catal. 2017, 69), 1H-X (X denote as metals) double-resonance MAS NMR experimental technique was developed and employed to study the interaction between the proton of Br?nsted acid sites and Zn or Ga species on Zn or Ga-modified ZSM-5 zeolite. The results gave strong evidence for the existence of synergic active sites on the metal-modified zeolites and the synergistic mechanism was revealed. Here, the proton-detected 1H/119Sn double-resonance correlation solid-state NMR method at high field and fast MAS speed is developed, in order to achieve direct observation of open Sn sites containing SnOH on Sn-β zeolites by correlating -OH groups with Sn atoms. Besides the closed Sn sites, two types of open Sn sites are directly identified on Sn-β and the 2J (119Sn–1H) constant of Sn-OH structure is determined to be 136 Hz (Figure 1). We also provide experimental evidence for the reversible interconversion between the closed and open Sn sites (Figure 2). On the basis of two-dimensional 1H/119Sn correlation MAS NMR results, the amount of open Sn site is determined by 119Sn MAS NMR spectra. The results presented in this work provide valuable insights into the nature of tin sites in Sn-β zeolite and open an avenue for the use of proton-detected solid-state NMR methods for characterization of metal sites (Ti、Zr) in zeolite catalysts.
This work is supported by the National Natural Science Foundation, the Chinese Academy of Sciences and the Science and Technology Department of Hubei Province. 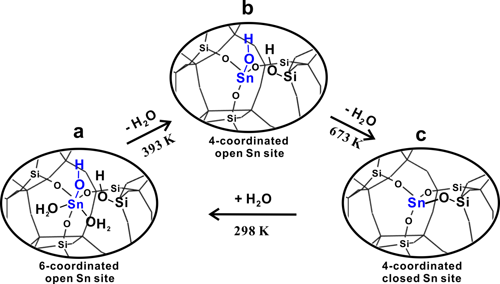
Figure 2. Proposed model for interconversion between open and closed Sn sites in Sn-β zeolite. |
|
|