|
|
Significant progress of Prof Tang and colleagues in structural dynamics of Ubiquitin |
| Author: |
|
Update time: |
2017-06-15 |
|
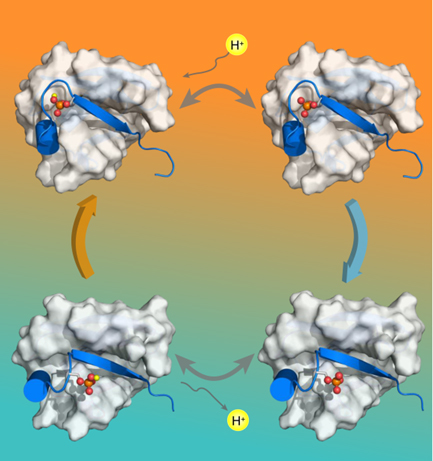 |
Prof Chun Tang and colleagues published a research paper titled “Ubiquitin S65 phosphorylation engenders a pH-sensitive conformational switch” at the Proceedings of the National Academy of Sciences (PNAS) on June 13. Their results reveal that four inter-exchange conformations are observed after phosphorylation at S65 of an ubiquitin monomer. The three-dimensional structures of these four conformations are solved, and the population of these conformations are regulated by pH. The further investigation using smFRET indicates that the conformational switch of the ubiquitin monomer would influence the quaternary structure of the polyubiquitin, resulting in interacting with various functional target proteins.
In 2014, phosphorylation at S65 of ubiquitin by PINK1 was reported, and it is known that phosphorylation of ubiquitin would activate PARKIN, leading to mitochondrial autophagy. However, the other functions of phosphorylated ubiquitin is not well clear so far. In this recently paper published in PNAS, Prof Tang and colleagues solved the high resolution structures of the relaxed conformation and the retracted conformation of the phosphorylation ubiquitin using NMR and other bio-physical methods. It shows that the relaxed conformation and the retracted conformation possess different pKa values, which are 7.2 and 5.8, respectively. Therefore, the phosphorylated ubiquitin would switch to the retracted conformation when pH increases, and it would switch to the relaxed conformation when pH decreases.
It is known that cellular pH varies among organelles and changes under physiological and pathological conditions. Ubiquitination and phosphorylation are the two most important protein posttranslational modifications and cell signals. Because ubiquitin is involved in myriad aspects of cell biology, a pH-sensitive conformational switch acquired upon S65 phosphorylation would allow phosphorylated ubiquitin to interact with different target proteins upon environmental cues. It would also enable cross-talk between ubiquitination and phosphorylation signals.
Prof Chun Tang and his team work on the structural dynamics of ubiquitin for long term, and they have already made a series of important publications. They discovered the non-covalent interaction of ubiquitin for the first time (Angew. Chem., 2012), and this interaction would regulate the quaternary structure of the polyubiquitin, and let the polyubiquitin interact with various target proteins through conformational selection mechanism. (eLife, 2015)
Dr Xu Dong and Dr Zhou Gong of Prof Tang’s team are the co-first authors of this paper. Dr Zhu Liu, whose PhD study were supervised by Prof Tang and who works at Zhejiang University, is the co-corresponding author of this paper. The work has been supported by the National Natural Science Foundation of China, HHMI and by the Chinese Ministry of Science and Technology. Link for the paper:http://www.pnas.org/content/early/2017/06/12/1705718114.abstract |
|
|